
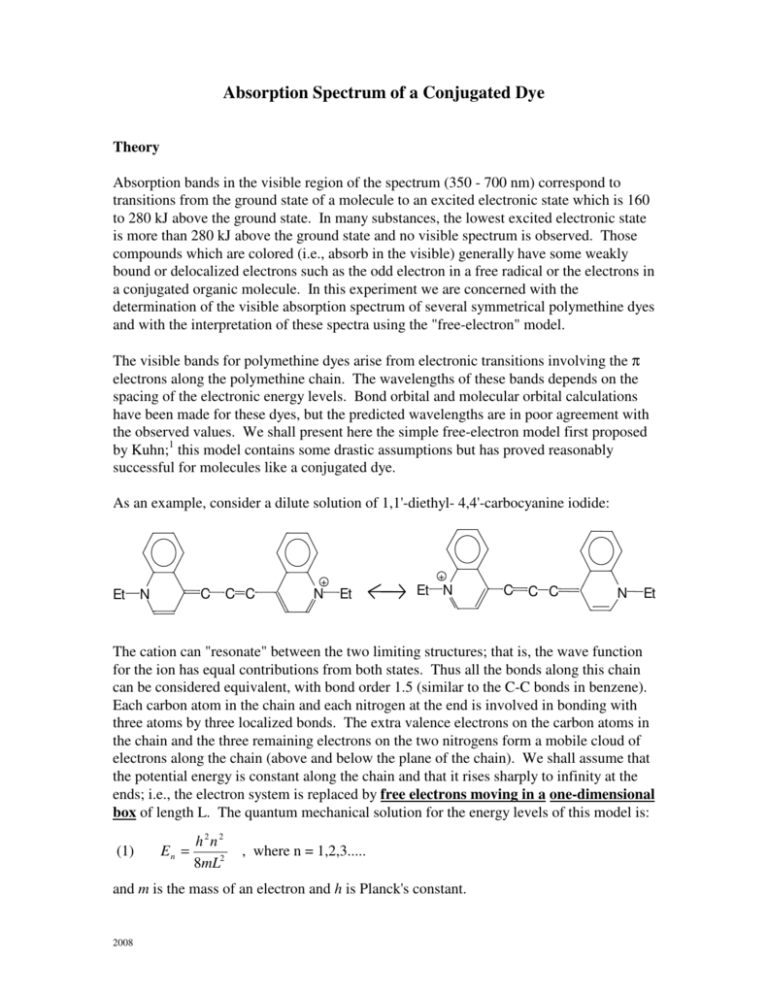
How many bonds long is that, and is that an integer multiple of the measured length (or a reasonably close approximation to an integer multiple of a bond length)? Compare that best guess and shape to what the HOMO and LUMO look like. You may need to change the number of alpha and beta phases that appear on one side of the molecule to get the longest length (flip a pi-bond over to get its phase out of the way of conjugation path). Particle in a Box : Absorption Spectrum of Conjugated Dyes Part A Recording the Spectra and Theoretical determination of max Theory Absorption bands in the visible region of the spectrum (350 - 700 nm) correspond to transitions from the ground state of a molecule to an excited electronic state which is 160 to 280 kJ above the ground state. That is, try alternating phase pairs of the pi orbitals and see which configuration(s) gives the longest length. Use what you know about MO's from organic chemistry and try assigning phases to the Kekule structures and see what you need to do to make the longest length of alternating (++)_(-) units. We all learn that conjugation is alternating double and single bonds right, but what is that here and how does it help? Although the conjugation method is limited too, it does offer a better approximation to a "length" since it starts with a good fragment (stable "vinylic like CH2=CH-" chunks). You are gonna say, "the whole thing is conjugated", but look closer. What is that thing, but the "conjugation length". So, can we make a better approximation without having to account for every electron? Yes, but we need to include those electrons in the molecule that are leading to the measured "characteristic length". So, the Kekule structure(s) (Lewis structure(s)) people start with to model the box length are very approximate. The molecular geometry provides the boundary conditions on the standing waves for the electrons, so people fix things by adding an extra length (fudge factor) to account for the discrepancy in the model. When one "arbitrarily" insists that the box is of a certain length, that is where any method fails. In eV this is an error of 20% (the 405 nm are wrong by about 5%), whereas your 200 nm would have been an error of 120%! When I use your box length and use 12 electrons (3 from the "left" benzene ring, 4 from the two double bonds to the "right" nitrogen" and 1 from the lone pair of this nitrogen), I come up with about 356 nm, which is not that far away as it sounds. where all the physical constants have been combined to give 63.7.Īs you have three carbon atoms between both nitrogens, you come up with 328 nm and when you include their "correction factor for polarizable end groups" of $\alpha = 0.675$, you come up with 405 nm, which is more than close to the experimental result. We are supposed to estimate this wavelength theoretically using this equation (energy change in a 1D box), where $N$ is the number of pi electrons. The experimental observed wavelength for this molecule is 425 nm.


 0 kommentar(er)
0 kommentar(er)
